Organic Chemistry 311
$25.00
Detailed topics of this course include nomenclature of organic compounds; structure and reactivity of alkanes, alkenes, alkynes, ethers, alcohols and alkyl halides; stereochemistry; nucleophilic substitution and β-elimination.
In Stock
Product Description
Excerpt:
Lecture Notes
Introduction — “These are the generations of the heavens and of the earth when they were created, in the day that the LORD God made the earth and the heavens, and every plant of the field before it was in the earth, and every herb of the field before it grew: for the LORD God had not caused it to rain upon the earth, and there was not a man to till the ground. But there went up a mist from the earth, and watered the whole face of the ground. And the LORD God formed man of the dust of the ground, and breathed into his nostrils the breath of life; and man became a living soul.” Genesis 2:4-7.
Organic Chemistry is simply the study of carbon-containing compounds. Other elements generally found in organic compounds are H, N, O, P, and S. What is so special about carbon that an entire section of chemistry is devoted to its compounds? The answer lies in the text above. The term organic literally means “derived from living organisms.” Just about all molecules from which living organisms are made contain carbon. Examples are proteins, vitamins, lipids, carbohydrates, nucleic acids and enzymes. The origin of the discipline of organic chemistry was the study of compounds extracted from living organisms and their natural products. Inorganic chemistry alternatively, referred to the study of inanimate objects and compounds that were derived from them. A term called vitalism was coined, which simply means that living organisms and their products needed a ‘vital force’ to create them. In 1828 however, a German chemist by the name of Friedrich Wohler converted ammonium cyanate (an inorganic compound) to urea (a major component in urine, i.e, an organic compound).
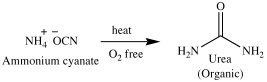
This reaction served to disprove the theory of vitalism as our predecessors understood it. In our day, chemists have synthesized many natural and unnatural organic compounds; medicines, plastics, pesticides, paints, and artificial body parts are all examples of organic compounds. But what about the ‘vital force?’ Why are some things animate and others inanimate and why are the materials from which they are made generally different? The answer lies in the same reaction discussed earlier. Ammonium cyanate and urea are constitutional isomers; that is, they have the same molecular formula (N2H4CO) but a different arrangement of the atoms (we will discuss this topic in detail in lecture 5). Hence, vitalism (the life force) cannot be found in the atoms themselves or their constitution, but in the source from which it was designed. “Hath not the potter power over the clay, of the same lump to make one vessel unto honor, and another unto dishonor?” Romans 9:21.
As we explore this branch of chemistry allow yourself the opportunity to learn how “clay” was used and is being used to support life.
About the Author
Glenn W.D. Phillips, Ph.D.
Assistant Professor (Oakwood since 2006)
Oakwood University Chemistry Department
Additional Information
| Isbn | 978-1-897403-68-6 |
|---|---|
| Print-type | Black / White |


 How to Write And Publish Your Church History
How to Write And Publish Your Church History
 Expressions of Love
Expressions of Love
 Coach Ridley’s Basketball Glory
Coach Ridley’s Basketball Glory
 Potluck Recipes
Potluck Recipes
Reviews
There are no reviews yet, would you like to submit yours?